Abstract
We have previously shown that Reactive Oxygen Species (ROS) promote Hematopoietic Stem and Progenitor Cell (HSPC) specification in the dorsal aorta, however, in the adult marrow it is established that HSPCs must maintain low levels of ROS to protect the stem cell pool against excessive cycling, differentiation, loss of stemness and apoptosis. We aim to elucidate the developmental timing and the mechanism by which HSCs transition from their initial dependence on ROS, to their precise regulation of it in the adult marrow. Since adult HSPCs regulate ROS by maintaining high levels of mitophagy, a process in which damaged mitochondria, which disproportionately generate ROS, are removed from the cell and recycled, we sought to define the initiation, regulation and impact of mitophagy in embryonic HSPC development.
Our recent data shows that oxidative stress limits HSPC numbers as early as their colonization of the secondary hematopoietic niche, the Caudal Hematopoietic Tissue (CHT). This is the zebrafish analog of the mammalian fetal liver in that it is the tissue to which the HSPCs migrate after emerging from the hemogenic endothelium of the dorsal aorta. Once in the CHT they mature and proliferate before migrating again to their terminal niche in the marrow. In contrast to published data showing that ROS promotes HSPC specification in the dorsal aorta, when ROS levels were increased in the embryo using a morpholino against PRDX1 we saw a decrease in runx1/c-myb expression in the CHT. Conversely, when ROS was decreased using the antioxidant N-acetyl cystine (NAC), there was an increase in HSPC marker expression. By live imaging of zebrafish embryos expressing a recently developed fluorescent transgenic mitophagy-reporter, Tg(ubi:mitoGR), we observed that mitophagy is low in the hemogenic endothelial tissue which gives rise to HSPCs, but is upregulated as HSPCs emerge and begin to migrate to the CHT, and is occurring at high levels at the developmental stage when ROS begins to become damaging to the HSPC population.
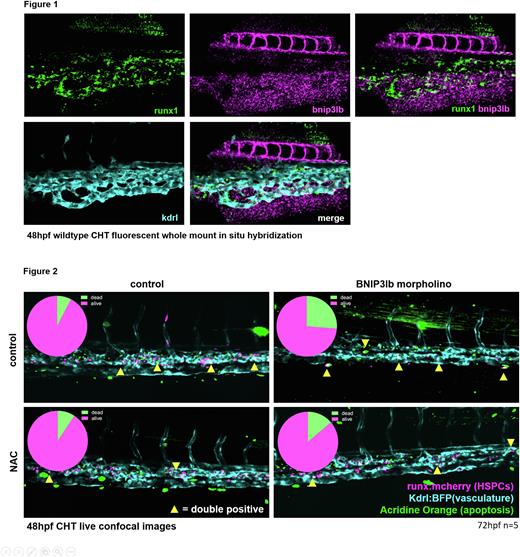
Our scRNAseq analysis and in situ-hybridization experiments allowed us to examine which genes and pathways were regulating the induction of mitophagy in these cells. These data show that the increase in mitophagy induction in the HSPC population at this timepoint coincides precisely with the initialization of expression of the bnip3lb mitophagy receptor gene. Fluorescent whole-mount in situ hybridization experiments demonstrated that bnip3lb is co-expressed with runx1 (a HSPC marker), in the CHT (figure 1), and, by examining our Tg(ubi:mitoGR) mitophagy reporter line after morpholino knock down of bnip3lb we confirmed that it is this gene which is driving mitophagy in HSPCs at this developmental stage. NIX, the murine homolog of bnip3lb, is responsible for developmentally regulated mitophagy in reticulocytes, lens fibers and macrophages, but has, thus far, had no reported role in maintaining HSPCs.
Functionally, mitophagy appears to be essential for the survival of the HSPC population in the CHT. Reducing mitophagy via morpholino directed knockdown of bnip3lb has no effect on the initial specification of HSPCs in the dorsal aorta, but significantly decreases HSPC marker expression in the CHT, as measured by runx1/c-myb in-situ hybridization, and quantified by CD41:GFP HSPC reporter expression. In contrast upregulating mitophagy, which can be done via a novel heat-shock driven ΔOTC transgenic line or via small molecule mitophagy induction, elevates HSPC numbers in the CHT.
Mechanistically, we found that bnip3lb knockdown increases detection of CellROX, a fluorescent dye which reports ROS levels, and increases the number of acridine orange+ apoptotic runx1+ HSPCs. Further, reducing ROS with antioxidant NAC reduces apoptosis and rescues the HSPC population (figure 2). We therefore propose that developmentally programmed mitophagy directed by bnip3lb is responsible for protecting proliferative embryonic HSPCs from the harmful effects of oxidative stress while the HSPC pool is expanding to seed lifelong hematopoiesis.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal